Government launch investigations over misuse of diabetes drug being used for weight loss
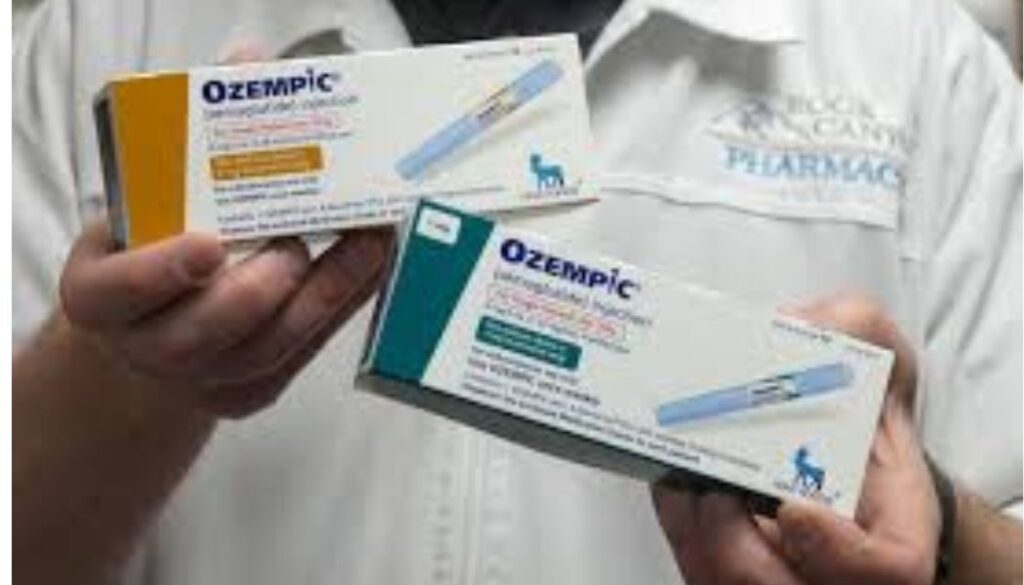
Government launch investigations over misuse of diabetes drug being used for weight loss
The government has launched investigations into the growing misuse of Ozempic, a diabetes drug that has recently gained popularity for weight loss.
Speaking on Thursday, June 5, Public Health Principal Secretary Mary Muthoni confirmed that the Pharmacy and Poisons Board (PPB) is actively examining the matter.
“The Pharmacy and Poisons Board (PPB) is currently looking into all concerns over the misuse of Ozempic and I’m sure we have a good report around it and the action that is being taken so that we can be there for the public good,” she said.
Muthoni raised concerns over increasing instances of self-medication and non-prescribed use of such drugs, warning against the culture.
“It would be important to note that we are on the move in regards to drugs abuse; if there is any use make sure that it is prescribed by a professional and ensure that you don’t walk into a supermarket or a pharmacy and buy drugs by yourself,” she added.
Ozempic is a prescription medication primarily used to treat type 2 diabetes.
Its active ingredient, semaglutide, is a glucagon-like peptide-1 (GLP-1) receptor agonist that works by mimicking the GLP-1 hormone to enhance insulin secretion.
While Ozempic is not officially approved for weight loss, it has become widely known for its ability to aid in reducing body weight.
This effect has led to growing interest among non-diabetic individuals, including celebrities and influencers, who have started using it off-label for cosmetic weight loss purposes.
This comes months after the Ministry of Health issued new directives after an assessment of aesthetic clinics and beauty spas across Nairobi, Mombasa, Nakuru, and Eldoret.
In a statement on Friday, February 7, Public Health PS Mary Muthoni said the ministry collaborated with health regulatory agencies to conduct the review.
She noted that the assessment uncovered alarming gaps in regulatory compliance, emergency preparedness and infection control measures.
According to the report, a total of 26 aesthetic clinics were assessed and out of these, only 20 were found to be compliant with licensing and operational standards.
On the other hand, three clinics had applied for registration and were awaiting approval, while another three were found to be operating illegally without any form of registration.
The report further revealed that a total of 102 beauty spas were inspected, with seven identified as offering invasive procedures such as micro-blading and micro-needling without proper registration or qualified personnel.
Tanzania shuts down X because of pornographic conten
ODM MP accuses Gachagua of planning DP Kindiki heckling in Nyeri
Salasya hits back at UDA Governor for mocking his presidential bid
Tanzania responds over Boniface Mwangi and Agather Atuhaire torture
DP Kindiki heckled in Nyeri as ‘Wantam’ slogans rent the air
Busia woman declares herself ‘Mary mother of Jesus’
The findings also indicated that 40% of the assessed facilities lacked essential emergency medical supplies, while 25% exhibited inadequate infection prevention and control measures.
In response to these findings, Muthoni has issued several directives aimed at tightening regulations and ensuring public safety.
She directed the Kenya Medical Practitioners and Dentists Council (KMPDC) to enforce the immediate closure of all unregistered aesthetic clinics operating illegally.
Facilities found to have inadequate infection prevention must comply with recommended standards within 30 days, while those lacking emergency medical supplies must establish and equip emergency trays within 60 days.
Muthoni also prohibited beauty and medical spas from offering or advertising invasive medical procedures unless they have been registered and licensed.
“All beauty and medical spas are prohibited from offering or advertising invasive medical procedures unless properly inspected, registered, and licensed as per the norms and standards,” the statement read.
Trump bans travel to US by nationals from 12 countries
Car prices to increase from July as KRA introduces new Current Retail Selling Price (CRSP)
KUCCPS to let students pursue their preffered university courses in policy shift
Gachagua’s DCP launches nationwide mobilisation programme to oust Ruto
Hospitals are in financial distress and receive low SHA payments; report
Follow us