Government recalls two nasal drugs from the market
The Ministry of Health on November 22, 2024, warned Kenyans against two drugs that have been deemed unsafe for usage
The Ministry of Health on November 22, 2024, warned Kenyans against two drugs that have been deemed unsafe for usage.
This is after the Pharmacy and Poisons Board ordered the recall of two nasal drops through a public alert statement.
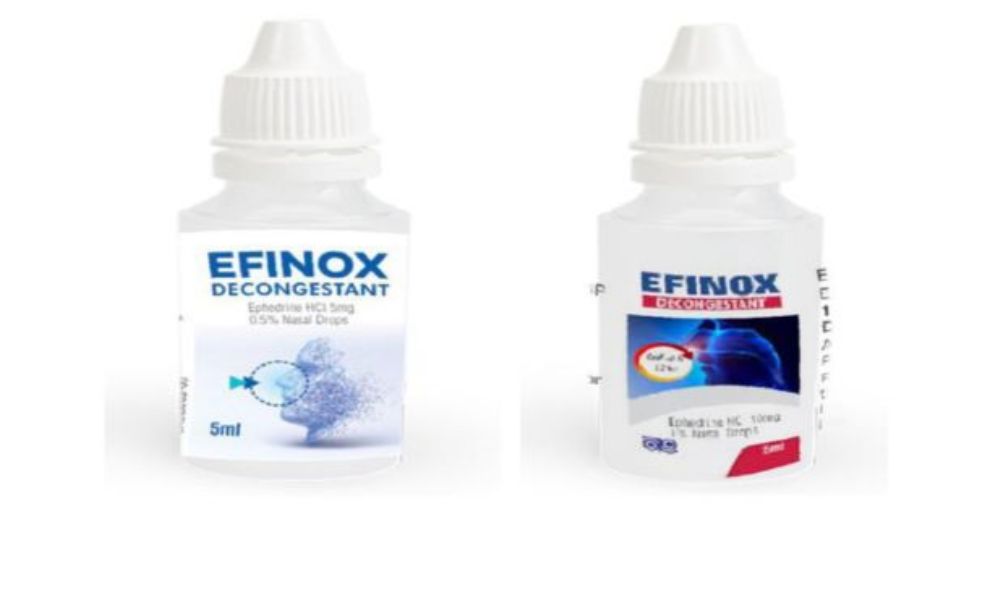
“The Pharmacy and Poisons Board has mandated the recall of Efinox 1% w/v Batch No.82979 and Efinox 0.5% w/v Batch No. 82978 manufactured by Laboratory and Allied Ltd, Kenya,” read the statement.
The Board stated that there was a mixup in the labeling and packing process of the two medicines that resulted in a disparity in the power of both drugs. As a result, this deemed the drugs unsafe for usage.
“The recall is being issued due to labeling mix-ups where the correct product was identified, but the wrong strength was applied. An investigation by Laboratory and Allied Ltd revealed that there was a likely mix-up between 0.5% w/v and 1% w/v strengths of the Efinox Nasal Drops during the labeling and packing process of the above batches,” said the board issue a directive to healthcare facilities and pharmacies to stop the distribution and issuance of the now-flagged batches.
In addition, it urged the aforementioned entities to return the nasal drugs.
IPOA Chairperson nominee Issack Hassan declares his net worth
Court suspends CA directive on IMEI numbers registration for mobile phones
Shock as another KCSE student dies under mysterious circumstances
NYS announces 108 job opportunities in Nairobi for its graduates; How to apply
Missing police officer found dead
Court quashes Safaricom Bonga Points expiry plan
“In light of this, the Board advises all pharmaceutical outlets, healthcare facilities, healthcare professionals, and members of the public to immediately stop the further distribution, sale, issuance, or use of the affected product batches.”
“The products should be returned to the nearest healthcare facility or respective suppliers,” added the statement.
The Board further advised Kenyans to raise attention to counterfeit drugs and harmful side effects resulting from the drugs.
This new development comes after a recent audit at the Board exposed a breach of ethics in supply chain integrity. The audit revealed that pharmaceutical distributors were supplying drugs to unlicensed pharmacies.
To combat this menace, plans are underway to take the 20 cases involving public and private hospitals as well as individuals to the Office of the Director of Public Prosecutions (ODPP).
Also read,
US Embassy issues directives to Kenyans seeking visa this December
Meg Whitman shares final message to Kenyans
Kenya supported Besigye arrest in Nairobi – Uganda’s ICT Minister
Gachagua-allied Senator blasts MPs after cancellation of Adani deals
US demands answers over Kizza Besigye abduction in Kenya
Matiang’i dragged to court over Sh6.5bn IDP cash
LSK wants costs, losses from cancelled Adani deals made public
Follow us