Government warns Kenyans against buying TWO commonly used drugs
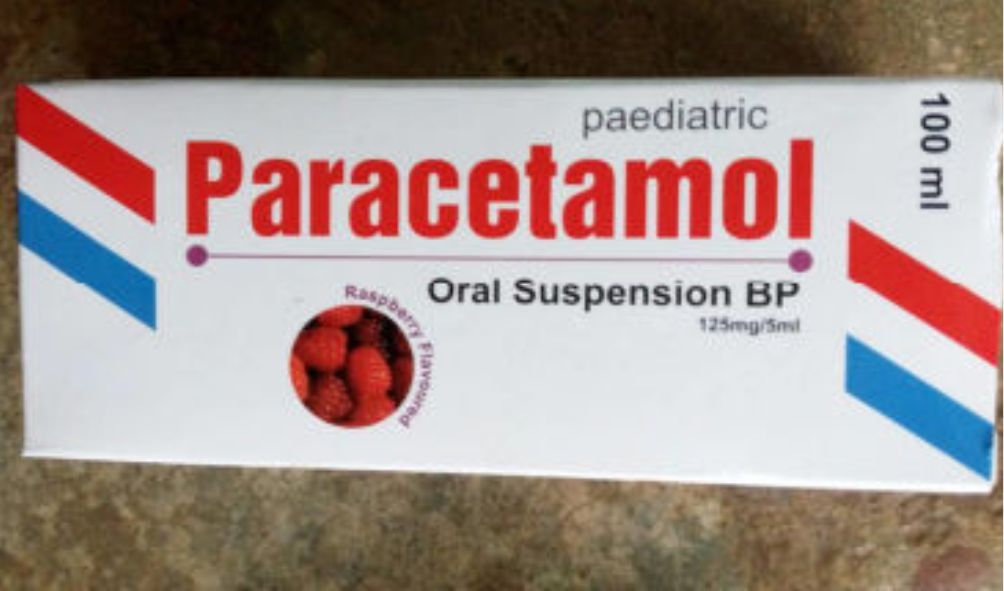
Government through Pharmacy and Poisons Board (PPB) cautions Kenyans against buying Iodixanol and the Paracetamol oral solution drugs
Government through Pharmacy and Poisons Board (PPB) cautions Kenyans against buying Iodixanol and the Paracetamol oral solution drugs.
The Pharmacy and Poisons Board (PPB) has issued an alert cautioning Kenyans against purchasing Iodixanol and the Paracetamol oral solution.
In a statement, the board confirmed that it had received worrying concerns and complaints in regard to the defects of Visipaque (Iodixanol) 320 mg/ml and Tramadol oral solution (Paracetamol oral solution, 120mg/5m).
“The Pharmacy and Poisons Board (PPB) has received several complaints regarding quality defects and suspected adverse events associated with suspected substandard and falsified (SF) batches of Visipaque (Iodixanol) 320 mg/mL vial” read part of the statement.
The Pharmacy and Poisons Board also cautioned pharmacies against distributing the two drugs.
“In an effort to safeguard public health and safety, healthcare facilities in possession of these affected product batches are urged to immediately quarantine the products and report to the Pharmacy and Poisons Board offices for further guidance and necessary action.”
Visipaque (Ioxidanol) is a medical contrast dye used during X-rays and CT scans to help doctors see blood vessels and organs more clearly.
It’s injected into your body briefly and then flushed out.
Paracetamol oral solution is a liquid medicine used to relieve pain and reduce fever.
It’s taken by mouth and is especially helpful for children or adults who have difficulty swallowing tablets.
The affected batches are Visipaque (Iodixanol) 320 mg/mL manufactured by GE Healthcare Ireland, Cork Ireland: Batch Number 15950809, 15944839, 15950792 and 15906117.
The other product is Visipaque (Iodixanol) 320 mg/mL manufactured by GE Healthcare (Shanghai) Co., Ltd, China: Batch Number 5389618, 15429745, 15444386, 15661498, 15904073, 15751274, 16017833, 16044911, 16085815, 16100415, 16107210 and 16177061.
Ruto responds after the death of EIGHT KDF soldiers in a chopper crash in Lamu
Shock as grade four pupil commits suicide inside classroom
Proposal to change presidential election system
State Department of Immigration to dispose uncollected 87,574 passports after 30 day ultimatum
EIGHT KDF personnel feared dead in chopper crash at Al-Shabaab stricken Boni forest, Lamu
According to the board, the medicine(Iodixanol) was smuggled into the country by unscrupulous traders who had not acquired approval.
Further, the Paracetamol oral solution manufactured by a Kenyan company failed to meet the prescribed market authorization requirements.
To that end, the board has advised Kenyans who have already purchased the drugs to return them to the nearest health facility.
The PPB strongly cautioned the public against engaging in any form of trade either in wholesale or retail, issuance, dispensing, or using it, warning of serious health repercussions for users who fail to heed the warning.
PPB vowed to take legal action against people found distributing the drugs.
“Any person found handling or distributing these products will be subject to legal action.”
PPB has since recalled the product in line with Section 3A(i) of the Pharmacy and Poisons Act (Cap. 244).
Also read,
CS Kuria sarcastically apologizes over fuel price remarks after Gachagua’s reprimand
FIVE Kenyans plead for help after enduring months of forced labour in a foreign country
President Biden heaps praise on Ruto over Haiti move
EU stands with French envoy in Niger over his tribulations under military
Screte push for Raila Odinga presidential candidacy in 2027
Follow us






